





.png)
October 2025 Update
The Health Resources and Services Administration (HRSA) has officially approved the 340B rebate process, marking a major milestone in the rollout of the Inflation Reduction Act’s (IRA) drug pricing provisions. This development underscores the importance for covered entities to understand how the rebate program will function — and what steps will be required to stay compliant and optimize reimbursement.
So far, HRSA has approved eight manufacturer plans covering nine of the ten IRA-selected drugs. Along with these approvals, HRSA released the final list of required data fields, which now include medical claims data — an important addition that expands reporting and verification requirements.
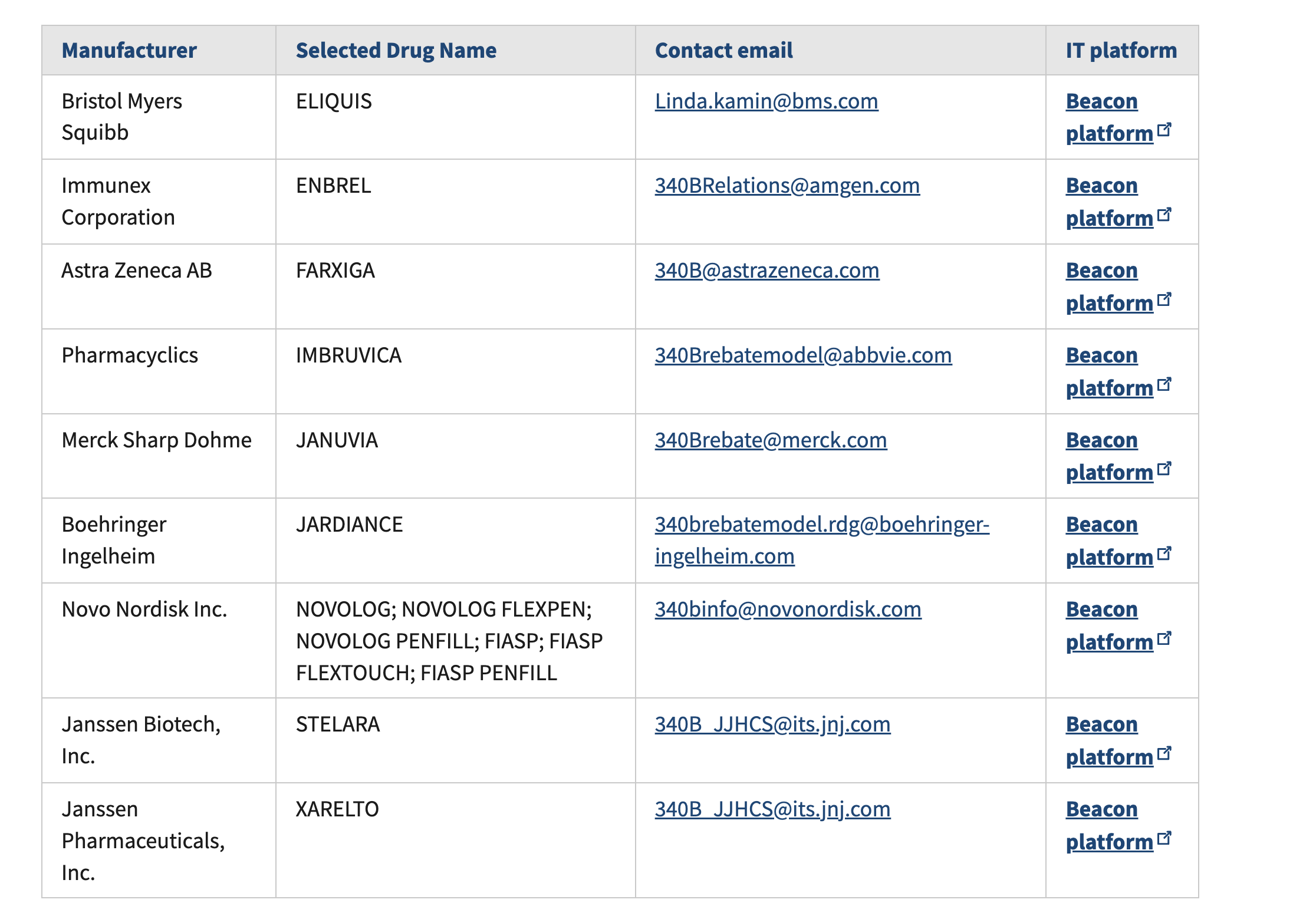
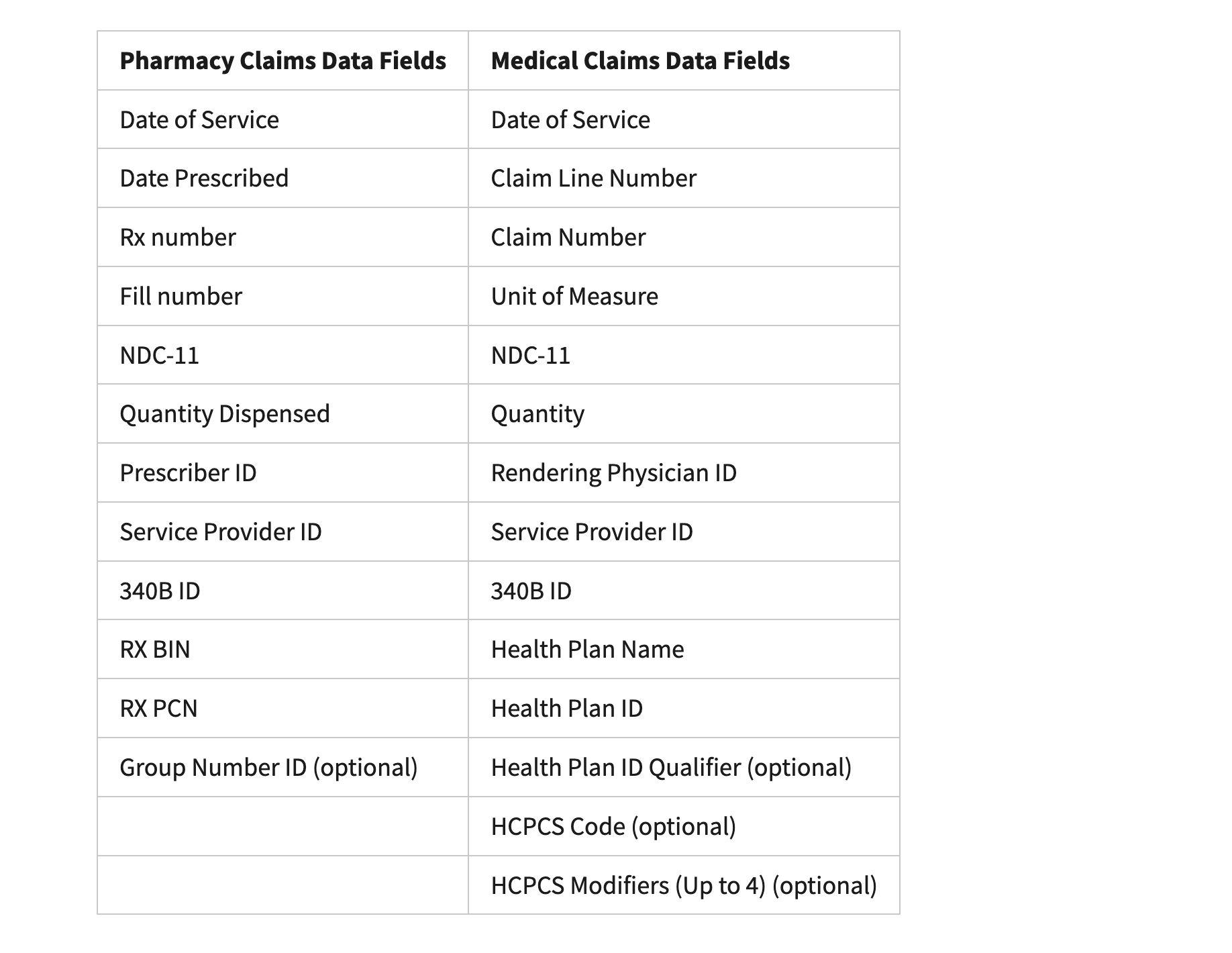
HRSA also confirmed that Beacon will serve as the central IT platform for all manufacturer rebate submissions and data validation. While this had been expected, the confirmation provides clarity for covered entities preparing to participate in the rebate process.
The RxTrail team has already begun planning for Beacon registration to ensure our clients are ready once submissions open. Beacon has released a Welcome Packet that outlines registration steps, which we’ll be sharing directly with clients to support a smooth onboarding process.
They have also updated the Beacon Rebate Model Claim Submissions with updated requirements. See Beacon Update
You can also stay up to date with the Beacon Learning Series.
Here are some helpful links:
With these updates, covered entities should begin reviewing internal data workflows to ensure they can meet the expanded data reporting requirements and integrate with Beacon’s system once live.
Our team is taking a proactive approach, already running analyses for our customers to determine how these changes may affect program operations and financial outcomes.
Another noteworthy item from this release was that all approved manufacturers will be using Beacon as their IT platform for processing rebates. As a next step, you will need to get registered with Beacon to ensure you’re ready once rebate submissions open. Learn more about the Beacon Registration Process.
RxTrail will continue monitoring updates from HRSA, manufacturers, and Beacon as rebate implementation begins. We’ll share additional insights and guidance as new timelines and data requirements are released.
If you’d like to talk with a team member on how this will impact your program schedule a call to review your program’s readiness.